Unveiling the Magic of Catalysis: Transforming Reactions for a Sustainable Future
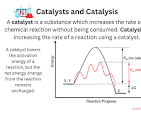
The Fascinating World of Catalysis
Catalysis is a fundamental process that plays a crucial role in various industries and scientific fields. It involves the acceleration or deceleration of a chemical reaction by a substance called a catalyst, without being consumed in the process. This phenomenon has revolutionised the way we produce chemicals, fuels, pharmaceuticals, and more.
One of the key benefits of catalysis is its ability to increase the efficiency of chemical reactions, leading to reduced energy consumption and waste generation. Catalysts work by providing an alternative reaction pathway with lower activation energy, making the desired reaction occur more quickly and at milder conditions.
There are two main types of catalysis: homogeneous and heterogeneous. In homogeneous catalysis, the catalyst is in the same phase as the reactants, while in heterogeneous catalysis, the catalyst is in a different phase. Each type has its advantages and applications depending on the specific reaction requirements.
Catalysts can be metals, metal oxides, enzymes, or even nanoparticles. They can be tailored and designed to exhibit specific properties that enhance their catalytic activity for particular reactions. The field of catalysis continues to evolve with ongoing research into new catalyst materials and mechanisms.
From industrial processes like petroleum refining and polymer production to environmental applications such as air pollution control and wastewater treatment, catalysis plays a vital role in addressing global challenges. Researchers are also exploring catalysis for sustainable energy production through processes like hydrogen fuel generation and carbon dioxide conversion.
In conclusion, catalysis is a fascinating field with far-reaching implications for society and the environment. Its ability to drive chemical transformations efficiently and selectively makes it an indispensable tool for modern science and technology. As research in catalysis advances, we can expect even more innovative solutions to emerge for a sustainable future.
Nine Advantages of Catalysis: Enhancing Efficiency, Sustainability, and Innovation in Chemical Processes
- Increases the rate of chemical reactions, leading to faster production processes.
- Reduces the energy required for reactions, resulting in lower energy costs.
- Enables the use of milder reaction conditions, reducing environmental impact.
- Provides greater control over reaction selectivity and product yield.
- Allows for recycling and reuse of catalysts, making processes more sustainable.
- Facilitates the production of high-quality products with fewer impurities.
- Opens up new possibilities for creating novel materials and compounds.
- Promotes efficiency in industrial processes by minimising waste generation.
- Contributes to the development of greener technologies with reduced carbon footprint.
Challenges and Drawbacks of Catalysis: Costs, Deactivation, Selectivity, Complexity, Impurities, and Environmental Concerns
- Some catalysts can be expensive to produce or acquire, adding to the overall cost of a catalytic process.
- Catalysts may lose their effectiveness over time due to deactivation or poisoning, requiring replacement and maintenance.
- Certain catalysts may have limited selectivity, leading to the formation of unwanted by-products in a reaction.
- The design and optimization of catalysts for specific reactions can be complex and time-consuming, hindering rapid implementation.
- In some cases, catalysts can introduce impurities into the final product, affecting its quality and purity.
- Environmental concerns arise from the use of certain catalysts that may generate toxic by-products or waste during catalytic processes.
Increases the rate of chemical reactions, leading to faster production processes.
Catalysis offers a significant advantage in increasing the rate of chemical reactions, resulting in faster production processes across various industries. By lowering the activation energy required for reactions to occur, catalysts facilitate quicker conversion of reactants into desired products. This acceleration not only boosts efficiency but also allows for streamlined and more cost-effective manufacturing processes, ultimately enhancing productivity and output in a timely manner.
Reduces the energy required for reactions, resulting in lower energy costs.
Catalysis offers a significant advantage by reducing the energy needed for chemical reactions, leading to decreased energy costs. By providing an alternative reaction pathway with lower activation energy, catalysts enable reactions to occur more efficiently and at milder conditions. This not only enhances the overall efficiency of industrial processes but also contributes to cost savings in energy consumption. As a result, catalysis plays a crucial role in improving the sustainability and economic viability of various industries by lowering their energy expenditure while maintaining high productivity levels.
Enables the use of milder reaction conditions, reducing environmental impact.
Catalysis offers the significant advantage of enabling the use of milder reaction conditions, thereby reducing the environmental impact of chemical processes. By lowering the required temperature and pressure for reactions to occur, catalysts help to minimise energy consumption and greenhouse gas emissions associated with traditional harsher reaction conditions. This not only contributes to more sustainable industrial practices but also promotes cleaner and greener production methods, aligning with the global efforts towards environmental conservation and reducing carbon footprints.
Provides greater control over reaction selectivity and product yield.
Catalysis offers a significant advantage by providing greater control over reaction selectivity and product yield. By using catalysts, chemical reactions can be directed towards producing the desired products with higher selectivity, reducing the formation of unwanted by-products. This precise control not only improves the efficiency of the reaction but also enhances the overall product yield. With catalysis, researchers and industries can tailor reactions to meet specific requirements, leading to more sustainable and cost-effective processes in various fields such as pharmaceuticals, materials science, and environmental remediation.
Allows for recycling and reuse of catalysts, making processes more sustainable.
Catalysis offers a significant advantage by enabling the recycling and reuse of catalysts, leading to more sustainable processes. This capability not only reduces the overall cost of production but also minimises waste generation and environmental impact. By recovering and reusing catalysts in multiple reaction cycles, industries can achieve greater efficiency and resource conservation, contributing to a more environmentally friendly approach to chemical manufacturing. The ability to recycle catalysts underscores the importance of catalysis in promoting sustainable practices across various sectors.
Facilitates the production of high-quality products with fewer impurities.
Catalysis plays a crucial role in facilitating the production of high-quality products with fewer impurities. By providing a specific reaction pathway that enhances selectivity and efficiency, catalysts enable the synthesis of desired compounds while minimising the formation of unwanted by-products. This precision and control in chemical reactions result in cleaner processes and superior product quality, making catalysis a key factor in ensuring the production of pure and refined substances across various industries.
Opens up new possibilities for creating novel materials and compounds.
Catalysis opens up new possibilities for creating novel materials and compounds by enabling the synthesis of complex molecules that would be challenging or impossible to produce through traditional chemical methods. Catalysts facilitate specific reactions that lead to the formation of unique structures and properties in materials, allowing researchers to explore uncharted territories in material science and chemistry. This pro of catalysis sparks innovation and creativity in the development of advanced materials with tailored functionalities, paving the way for exciting breakthroughs in various industries and scientific disciplines.
Promotes efficiency in industrial processes by minimising waste generation.
Catalysis promotes efficiency in industrial processes by minimising waste generation. By providing an alternative reaction pathway with lower activation energy, catalysts enable chemical reactions to occur more rapidly and at milder conditions, leading to higher yields of desired products and reduced by-product formation. This not only enhances the overall productivity of industrial operations but also contributes to environmental sustainability by decreasing the amount of waste produced during manufacturing processes. The ability of catalysis to streamline reactions and minimise waste generation underscores its significant role in driving cleaner and more resource-efficient industrial practices.
Contributes to the development of greener technologies with reduced carbon footprint.
Catalysis contributes significantly to the development of greener technologies with a reduced carbon footprint by enabling more efficient and environmentally friendly chemical processes. By lowering the energy requirements and enhancing selectivity in reactions, catalysts play a crucial role in reducing waste generation and improving overall process efficiency. This leads to the creation of cleaner production methods that help mitigate the impact of industrial activities on the environment, ultimately supporting sustainability efforts and promoting a more eco-friendly approach to technology development.
Some catalysts can be expensive to produce or acquire, adding to the overall cost of a catalytic process.
Some catalysts can be expensive to produce or acquire, which presents a significant drawback in catalysis. The cost of high-quality catalyst materials can escalate the overall expenses of a catalytic process, making it economically challenging for industries to adopt catalysis on a large scale. This financial barrier may hinder the widespread implementation of catalytic technologies, especially in sectors where cost-effectiveness is a critical factor. Finding cost-effective alternatives or developing more efficient catalyst synthesis methods are essential steps to address this con and make catalysis more accessible and sustainable in various applications.
Catalysts may lose their effectiveness over time due to deactivation or poisoning, requiring replacement and maintenance.
One significant drawback of catalysis is the potential loss of effectiveness in catalysts over time due to deactivation or poisoning. Deactivation can occur as a result of changes in the catalyst’s structure or composition during repeated use, leading to a decrease in its catalytic activity. Additionally, catalysts may be poisoned by contaminants or unwanted by-products present in the reaction environment, further reducing their efficiency. This necessitates regular replacement and maintenance of catalysts, adding to the cost and complexity of catalytic processes. Efforts are ongoing in research and development to mitigate these issues and improve the durability and stability of catalysts for long-term use.
Certain catalysts may have limited selectivity, leading to the formation of unwanted by-products in a reaction.
One significant drawback of catalysis is the potential lack of selectivity exhibited by certain catalysts, which can result in the formation of unwanted by-products during a chemical reaction. This issue can complicate the purification process and reduce the overall efficiency of the reaction, leading to increased waste generation and decreased yield of the desired product. The presence of unwanted by-products can also impact the economic viability and environmental sustainability of catalytic processes, highlighting the importance of developing highly selective catalysts to minimise side reactions and enhance overall efficiency in chemical transformations.
The design and optimization of catalysts for specific reactions can be complex and time-consuming, hindering rapid implementation.
The design and optimisation of catalysts for specific reactions can present a significant challenge in the field of catalysis. This complexity often arises from the need to tailor catalyst properties to suit the unique requirements of each reaction, which can be a time-consuming and intricate process. The extensive research and testing required to develop effective catalysts for desired chemical transformations may hinder rapid implementation of catalytic processes in various industries. This con highlights the importance of continued innovation and collaboration in catalysis research to overcome these obstacles and streamline the development of efficient catalysts for practical applications.
In some cases, catalysts can introduce impurities into the final product, affecting its quality and purity.
In certain instances, a drawback of catalysis is the potential for catalysts to introduce impurities into the end product, thereby compromising its quality and purity. Despite their intended role in facilitating reactions, catalysts may inadvertently interact with reactants in ways that lead to the formation of undesired by-products or contaminants. This issue poses a challenge in industries where product purity is paramount, as any impurities introduced by the catalyst can impact the overall quality of the final output. Careful selection and design of catalysts are essential to mitigate this con and ensure that catalytic processes yield high-quality products free from unwanted impurities.
Environmental concerns arise from the use of certain catalysts that may generate toxic by-products or waste during catalytic processes.
Environmental concerns arise from the use of certain catalysts in catalytic processes due to the potential generation of toxic by-products or waste. While catalysis offers numerous benefits in terms of efficiency and selectivity, some catalysts may inadvertently produce harmful substances that can have detrimental effects on ecosystems and human health. It is crucial for researchers and industry professionals to consider the environmental impact of catalysts and strive to develop more sustainable alternatives that minimise waste and pollution while maintaining high performance in chemical reactions.
Latest articles
- Volunteer Near Me on Christmas Day: Spread Joy and Give Back to Your Community
- Spreading Festive Cheer: The Joy of Volunteering on Christmas Day
- Exploring the Diversity: Understanding Different Types of Stem Cells
- Exploring Different Types of Qualitative Research Approaches
- Exploring the Impact of Trisha Greenhalgh’s Work on Healthcare Research
Latest comments
Archive
- December 2025
- November 2025
- October 2025
- September 2025
- August 2025
- July 2025
- June 2025
- May 2025
- April 2025
- March 2025
- February 2025
- January 2025
- December 2024
- November 2024
- October 2024
- September 2024
- August 2024
- July 2024
- June 2024
- May 2024
- April 2024
- March 2024
- February 2024
- January 2024
- December 2023
- November 2023
- October 2023
- September 2023
- August 2023
- July 2023
- June 2023
- May 2023
- April 2023
- March 2023
Categories
- 2020
- 2021
- academic search
- academic search engines
- activate learning
- active learn
- adobe
- adult
- adult education center
- advanced materials
- alison
- alzheimer's
- alzheimer's research
- amazon
- animal
- animal charity
- apa
- apa style
- apple
- applicant
- applied innovation
- architecture
- art
- astrophysics
- aws
- bandlab
- bbc
- bioengineering
- bioinformatics
- bloomberg
- breakthrough innovation
- british school
- building construction
- cancer
- cancer research
- cancer uk
- catholic school
- certificate programs
- ceus
- chemistry
- child
- christmas day
- city
- closed innovation
- cognitive psychologist
- cognitive science
- college
- colleges
- community health
- computer science
- computer studies
- computing
- construction
- consulting jobs
- content analysis
- cross industry innovation
- d innovation
- defense innovation board
- degree courses
- department for education
- department of technical education
- disruptive innovation
- distance learning centre
- doblin
- doctor degree
- drivers ed
- driving classes
- early childhood education
- early learning
- early learning center
- early years
- ece
- education
- education authority
- education city
- education jobs
- educational psychologist
- educational systems
- elementary
- elementary education
- elementary teacher
- engineering
- english
- environment
- environmental health
- environmental science
- epsrc
- eu
- europe
- european
- european journal
- european journal of marketing
- exam
- example
- examples
- experimental research
- exploratory research
- finance
- finance innovation
- find a phd
- focus group
- food bank
- food pantry
- four
- gap
- get into teaching
- google academic
- google research
- google scholar
- google search
- googled
- graduate
- graduate programs
- graduate student
- green construction
- green innovation
- harvard
- head start
- health
- healthy life
- high school
- higher world
- hospital
- idea solutions
- iep
- incremental innovation
- indoor
- industrial
- industrial building
- industry innovation and infrastructure
- innovate solutions
- innovation
- innovation department
- innovation engine
- innovation software
- innovative consultancy
- innovative management solution
- innovative software services
- innovative technology solutions
- interdisciplinary studies
- international food research journal
- international marketing
- interpretative phenomenological analysis
- ipa
- journal article
- journal articles
- journal of business
- journal of international
- journal of marketing
- journal of marketing research
- journal scholar
- journalism
- jugaad innovation
- khan academy
- learn
- learning
- learningonline
- lego
- liberal arts
- literature
- loan
- longitudinal research
- longitudinal study
- ma
- maed
- management
- management innovation
- market innovation
- marketing
- marketing research
- master of education
- masters
- math
- math teacher
- maths
- mckinsey
- medical
- medical research council
- medicine
- meeting
- memoir
- memoirs
- mendeley
- mental well being
- mental wellbeing
- method
- methods
- microsoft
- microsoft office
- military academy
- ministry of education
- modular innovation
- montessori
- mooc
- mrc
- ms
- music
- music teacher
- narrative analysis
- neom
- neuroscience
- nih
- nursing
- observational research
- office 365
- online academy
- online degree
- online learning academy
- online programs
- online school
- online university
- onlinestudies
- open
- open learning
- organizational innovation
- paper
- participant observation
- pe teacher
- pharmaceutical
- phd
- philosophy
- physical education
- physics
- pi research
- postgraduate
- postgraduate courses
- preschool teacher
- preschools near me
- primary education
- process innovation
- product development and management
- product innovation
- production management
- programming course
- programming courses
- psychology
- public health
- qualitative analysis
- qualitative data
- qualitative research journal
- radical innovation
- reference manager
- reggio emilia
- religious education
- research
- research article
- research gates
- research paper
- research paper writing
- research scholar
- researchgate
- rspca
- scholar google
- scholare
- scholarly journal
- school
- schools
- science
- search engines
- secondary
- secondary data
- secondary education
- sen teacher
- service innovation
- skills
- sociology
- software innovation
- special education
- special education teacher
- sped
- spiritual well being
- spiritual wellbeing
- stem cell technologies
- stem cell technology
- strategic management
- student
- student finance england
- student loan
- student loans
- survey research
- sustaining innovation
- synonym
- teach
- teachcomputing
- teacher
- teacher development
- teacher jobs
- teachers
- teaching
- tech schools
- ted talk
- ten
- tertiary education
- testing
- texes test
- thematic analysis
- three
- times higher
- top 100
- topuniversities
- train
- training
- ttra
- types of correlation
- uk
- uk edge
- Uncategorized
- universities
- university
- university student
- university times
- urban design
- vocational school
- volunteer abroad
- warwick
- web of science
- week
- wellbe
- work and wellbeing
- work on wellbeing
- work well being
- world first
- world university
